ALS Biologics
The ALScell™ (aka JadiCell™) is unique in that it possesses features of mesenchymal stem cells, however, ALScells outperform these cells in terms of a) enhanced growth factor production; b) augmented ability to secrete exosomes; and c) superior angiogenic
and neurogenic ability.
ALScells
Protection and Regeneration of Neurological Function by Using Stem Cells.
Publication No.: 20220125852
Type: Application
Filed: October 27, 2021
Publication date: April 28, 2022
Umbilical Cord Derived Regenerative and Immune Modulatory Stem Cell Populations
Publication number: 20230226119
Type: Application
Filed: Dec 2, 2022
Publication Date: Jul 20, 2023
In one embodiment, cells of the invention may be utilized for treatment of Amyotrophic lateral sclerosis (ALS). This is a progressive neurodegenerative condition causing muscular atrophy and death within 3–5 years after its onset [1]. In the majority of patients (90%) the cause of ALS is idiopathic, however in about 10% of the patients a familial form of the disease is presented [2]. Specific muscular degeneration is exclusive to motor neurons and begins focally and spreads, leading to weakness of limb, respiratory, and bulbar muscles. Immediately preceding death, there is a near total loss of limb and respiratory function, as well as a loss of the ability to chew, swallow, and speak.
In the USA, ALS is defined as an “orphan disease,” with approximately 2 per 100,000 new cases per year and a prevalence of about 5 per 100,000 total cases each year [3]. In the United States [4] and Europe [5], ALS is diagnosed in about 1 in 500 to 1 in 1,000 adult deaths, implying that 500,000 people in the United States will develop this disease in their lifetimes. About 10% of ALS cases are inherited, usually as dominant traits [6]. Both familial ALS (fALS) and sporadic ALS (sALS) can develop concurrently with frontotemporal lobar dementia (FTLD). By contrast with the dementia of Alzheimer disease (AD), in which the cardinal finding is memory loss, FTLD is characterized by behavioral changes and progressive aphasia, sometimes accompanied by movement disorders.
While AD involves prominent pathology in the hippocampus, the essential finding in FTLD is, as the name suggests, early atrophy of the frontal and temporal lobes. Four recurring themes have emerged from the pathological analysis of autopsied cases with sALS, fALS, or ALS-FTLD with diverse genetic causes. First, the motor neuron death usually entails deposition of aggregated proteins, often ubiquitinated and predominantly cytoplasmic. Second, in ALS, the levels and functions of RNA and RNA-binding proteins are abnormal. Aggregates of protein and RNA are detected both in motor neurons and non-neuronal cells, such as astrocytes and microglia. Third, most cases entail some disturbance of neuronal cytoskeletal architecture and function. Additionally, in almost all cases, motor neuron death is influenced by non-neuronal cells, including oligodendroglia and cells involved in neuroinflamation (e.g., astroglia and microglia).
We incorporate by reference trials of other types of MSC in order to provide guidance for one of skill in the art how to administer the cells of the current invention, and how to designed clinical trials based on previous types of cell therapies.
One of the first clinical interventions using mesenchymal stem cells in ALS was a report by Mazzin et al [7], who treated ALS patients with bone marrow ex vivo expanded MSC. Specifically, bone marrow collection was performed according to the standard procedure by aspiration from the posterior iliac crest. Ex vivo expansion of mesenchymal stem cells was induced according to Pittenger’s protocol [8].
The cells were suspended in 2 ml of autologous cerebrospinal fluid and transplanted into the spinal cord by a micrometric pump injector. No patient manifested major adverse events such as respiratory failure or death. Minor adverse events were intercostal pain irradiation (4 patients) which was reversible after a mean period of three days after surgery, and leg sensory dysesthesia (5 patients) which was reversible after a mean period of six weeks after surgery. No modification of the spinal cord volume or other signs of abnormal cell proliferation were observed. The authors concluded by stating that it appears that the procedures of ex vivo expansion of autologous mesenchymal stem cells and of transplantation into the spinal cord of humans are safe and well tolerated by ALS patients.
The same group reported 3 year follow up of the initial patients treated. Seven patients affected by definite ALS were enrolled in the study and two patients were treated for compassionate use. No patient manifested major adverse events such as respiratory failure or death. Minor adverse events were intercostal pain irradiation and leg sensory dysesthesia, both reversible after a mean period of 6 weeks. No modification of the spinal cord volume or other signs of abnormal cell proliferation were observed. A significant slowing down of the linear decline of the forced vital capacity was evident in four patients 36 months after MSCs transplantation [9]. An additional two studies where performed by the same group on 10 and 19 patients. The longest observation of treated patients was performed at 9 years after treatment. No long term adverse effects were detected and marginal therapeutic effects were seen [10, 11].
A study by an independent group evaluated the safety of two repeated intrathecal injections of autologous bone marrow (BM)-derived mesenchymal stromal cells (MSCs) in ALS patients. Eight patients with definite or probable ALS were enrolled. After a 3-month lead-in period, autologous MSCs were isolated two times from the BM at an interval of 26 days and were then expanded in vitro for 28 days and suspended in autologous cerebrospinal fluid. Of the 8 patients, 7 received 2 intrathecal injections of autologous MSCs (1 × 10(6) cells per kg) 26 days apart. Clinical or laboratory measurements were recorded to evaluate the safety 12 months after the first MSC injection.
The ALS Functional Rating Scale-Revised (ALSFRS-R), the Appel ALS score, and forced vital capacity were used to evaluate the patients’ disease status. One patient died before treatment and was withdrawn from the study. The death was not study related, and was attributable to natural progression of disease. With the exception of that patient, no serious adverse events were observed during the 12-month follow-up period. Most of the adverse events were self-limited or subsided after supportive treatment within 4 days. Decline in the ALSFRS-R score was not accelerated during the 6-month follow-up period. Two repeated intrathecal injections of autologous MSCs were safe and feasible throughout the duration of the 12-month follow-up period [12].
A subsequent study from Belarus utilized autologous mesenchymal stem cells were injected intravenously (intact cells) or via lumbar puncture (cells committed to neuronal differentiation). Evaluation of the results of cell therapy after 12-month follow-up revealed slowing down of the disease progression, as assessed by ALSFRS-R score was observed in 10 patients that were treated with cells. In comparison, in a control group that was matched for age and disease status, no slowing down of progression was observed. The Control group consisted of 15 patients. The study reported no adverse effects associated with administration of mesenchymal stem cells intravenously or intrathecally [13].
Although the above clinical studies support a possible benefit for reducing progression of ALS, as determined by slowing down advancement of disease as quantified by measures such as ALSFRS-R score, none of the studies reversed the disease progression. One possible reason is that when the stem cells where introduced via lumbar injection (intrathecally), the cells hypothetically would tend to sink downwards rather than ascending to the brain and cervical and thoracic spinal cord. Therefore, a study by Baek et al. [14], assessed the ability to utilize intraventricular injections directly into the brain by using an Ommaya reservoir to administer cells. The Ommaya reservoir is catheter system that is typically used for the delivery of drugs directly into the ventricles of the brain. It consists of a catheter in one lateral ventricle attached to a reservoir implanted under the scalp. It is typically used to treat brain tumors, leukemia/lymphoma or leptomeningeal disease, as well as for intracerebroventricular (ICV) injection of morphine [15].
Others have previously used the Ommaya reservoir to deliver cell therapy into the brain. To give an indication of the relative safety of this approach, in one study in glioma patients, autologous tumor infiltrating lymphocytes that were expanded ex vivo we administered in 6 patients by use of the Ommaya reservoir. One patient had complete response, 2 had partial responses, and 3 succumbed to disease. Most interestingly, no serious adverse effects were noted, despite the fact that activated lymphocytes were directly injected into the brain, an area typically classified as very sensitive in inflammation [16]. With the rational this, and other studies have successfully administered cells in the brain [17, 18, 19], and mesenchymal stem cells are generally considered anti-inflammatory, Baek et al attempted to adopt this procedure for use in ALS in a patient.
Bone marrow mesenchymal stem cells were isolated from the bone marrow of a male patient with ALS who underwent insertion of an Ommaya reservoir. Expanded MSCs (hBM-MSCs: dose of 1 X 106 cells/kg) were suspended in autologous CSF and directly transplanted into the ALS patient’s lateral ventricle via the Ommaya reservoir. Clinical, laboratory, and radiographic evaluation of the patient revealed no serious adverse effects related to the stem cell therapy. The authors concluded that intraventricular injection with an optimized number of cells is safe, and is a potential route for stem cell therapy in patients with ALS. Intraventricular injection via an Ommaya reservoir makes repetitive injection of stem cells easy and reliable even in far advanced ALS patients. Unfortunately, no discussion on impact on disease progression was given in the publication.
In another attempt to increase therapeutic efficacy of mesenchymal stem cells in ALS, researchers have explored in vitro means of augmenting neurotrophic factor production by manipulation of culture conditions. A series of studies from the Hadassah Medical Center in Jerusalem, Israel attempted to treat ALS by in vitro manipulated MSC that are validated to produce higher amounts of neurotrophic factors. In the studies, all patients were followed up for 3 months before transplantation and 6 months after transplantation. In the phase 1/2 part of the trial, 6 patients with early-stage ALS were injected intramuscularly (IM) and 6 patients with more advanced disease were transplanted intrathecally (IT).
In the second stage, a phase 2a dose-escalating study, 14 patients with early-stage ALS received a combined IM and IT transplantation of autologous MSC-NTF cells. It was reported that among the 12 patients in the phase 1/2 trial and the 14 patients in the phase 2a trial aged 20 and 75 years, the administration of mesenchymal stem cells was found to be safe and well tolerated over the study follow-up period. Most of the adverse effects were mild and transient, not including any treatment-related serious adverse event. The rate of progression of the forced vital capacity and of the ALSFRS-R score in the IT (or IT+IM)-treated patients was reduced (from -5.1% to -1.2%/month percentage predicted forced vital capacity, P < .04 and from -1.2 to 0.6 ALSFRS-R points/month, P = .052) during the 6 months following MSC-NTF cell transplantation vs the pretreatment period. Of these patients, 13 (87%) were defined as responders to either ALSFRS-R or forced vital capacity, having at least 25% improvement at 6 months after treatment in the slope of progression.
A subsequent report on MSC-NTF cells described observations after these cells where delivered by combined intrathecal and intramuscular administration to participants with amyotrophic lateral sclerosis (ALS) in a phase 2 randomized controlled trial. The study enrolled 48 participants randomized 3:1 (treatment: placebo). After a 3-month pretransplant period, participants received 1 dose of MSC-NTF cells (n = 36) or placebo (n = 12) and were followed for 6 months. CSF was collected before and 2 weeks after transplantation.The study met its primary safety endpoint. The rate of disease progression (Revised ALS Functional Rating Scale [ALSFRS-R] slope change) in the overall study population was similar in treated and placebo participants. In a prespecified rapid progressor subgroup (n = 21), rate of disease progression was improved at early time points (p < 0.05).
To address heterogeneity, a responder analysis showed that a higher proportion of treated participants experienced ≥1.5 points/month ALSFRS-R slope improvement compared to placebo at all time points, and was significant in rapid progressors at 4 and 12 weeks (p = 0.004 and 0.046, respectively). CSF neurotrophic factors increased and CSF inflammatory biomarkers decreased in treated participants (p < 0.05) post-transplantation. CSF monocyte chemoattractant protein-1 levels correlated with ALSFRS-R slope improvement up to 24 weeks (p < 0.05). Thus the authors summarized that a single-dose transplantation of MSC-NTF cells is safe and demonstrated early promising signs of efficacy. This establishes a clear path forward for a multidose randomized clinical trial of intrathecal autologous MSC-NTF cell transplantation in ALS [20].
In conclusion, for the use of the current invention in the treatment of ALS, there are means of enhancing efficacy. The cells of the current invention allow for a) increasing frequency of dosing, in part because the cells are smaller in size than typical MSC, higher number of cells may be given without fear of lung pathology.; b) further augmentation of mesenchymal stem cell regenerative activity through culture conditions or gene manipulation, for example, addition of neurotrophic factors in vitro to enhance activity may be performed; c) the use of other growth/neurotrophic factors as adjuvants. For example, it is known that endogenous neuronal stem cells exist, whose activity may be modified by administration of various compounds, some compounds already clinically available.
Well-known examples of approved drugs that augment endogenous neural stem cell activity include lithium [21, 22], valproic acid [23], and human chorionic gonadotropin [24], these agents are disclosed for use within the context of the current invention. Interestingly, the stem cell modifier combination of lithium and valproic acid was already assessed on its own in a small trial which suggested some possible efficacy. The study recruited 18 patients that were treated with the combination and compared them to 31 controls that were carefully paired by age, gender, evolution rate and time of the disease, who never received treatment with lithium and/or valproate. Assessment of disease by ALSFRS-R was performed before treatment (baseline), 1 month after treatment, and every 4 months until the outcome (death or an adverse event). The investigators reported that lithium and valproate cotreatment significantly increased survival, and this treatment also exerted neuroprotection in our patients because all biochemical markers reached levels normal levels in the ALS patients that were treated. The biochemical markers were Cu/Zn superoxide dismutase and glutathione peroxidase activity, and reduced glutathione [25].
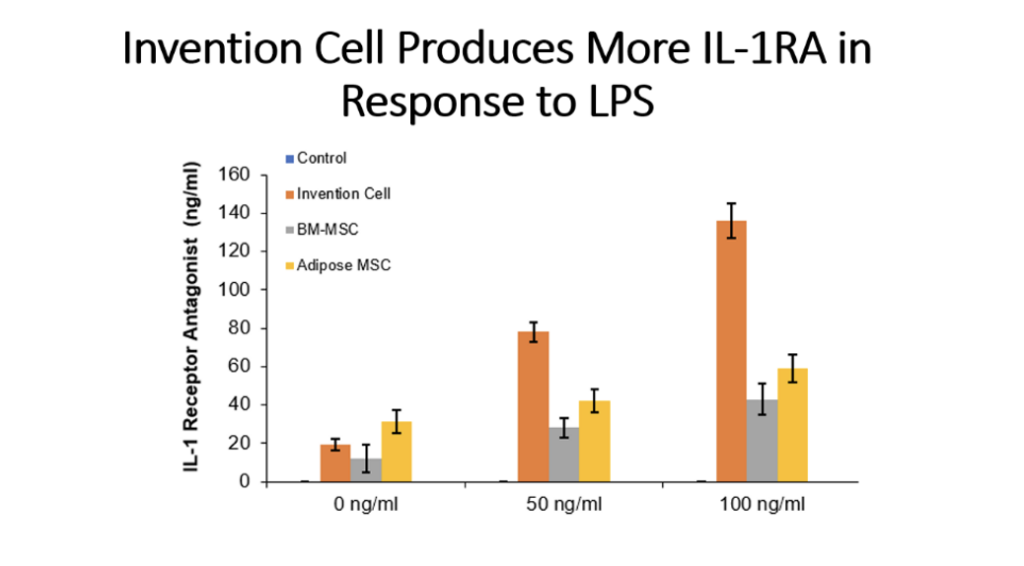
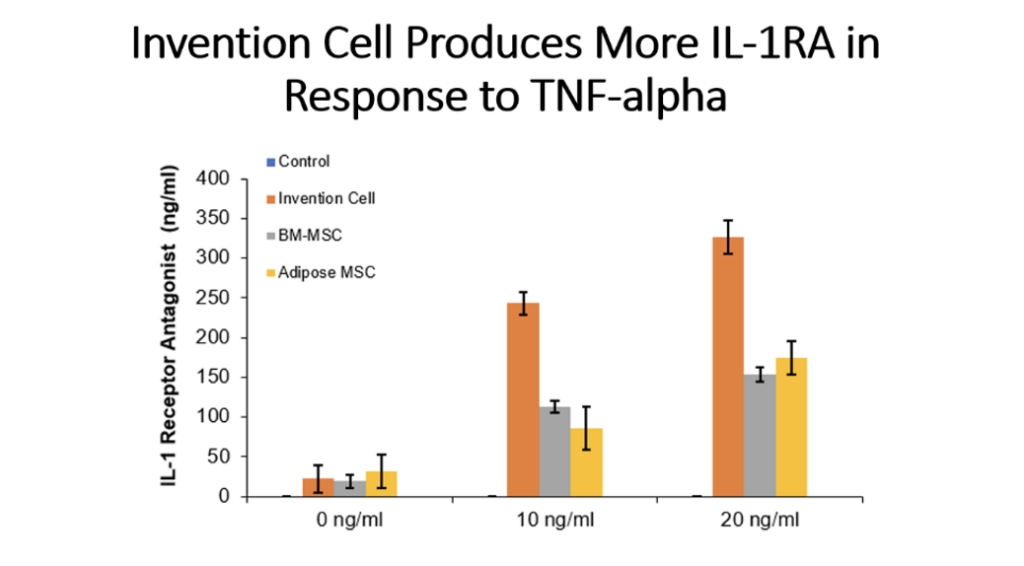
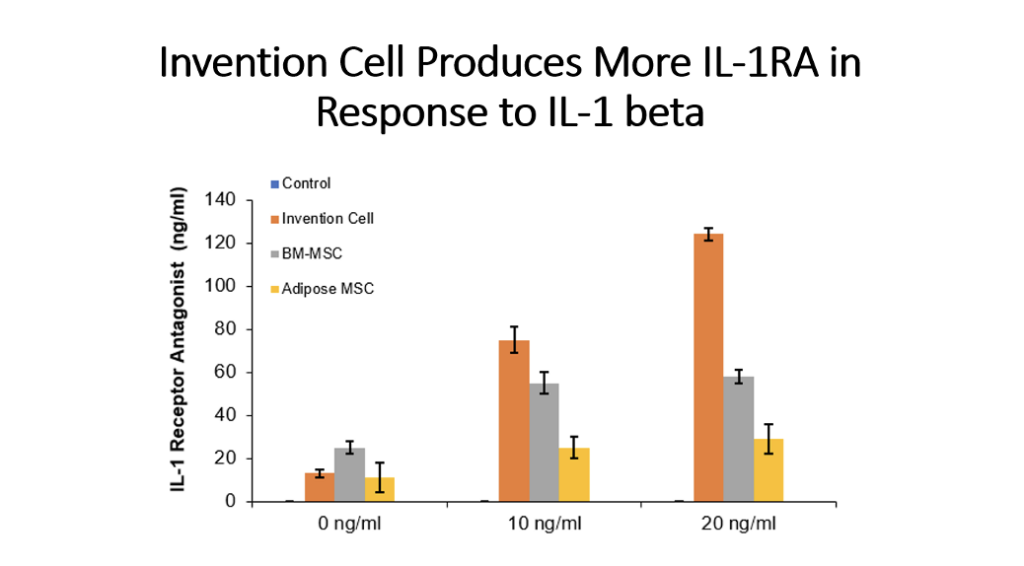
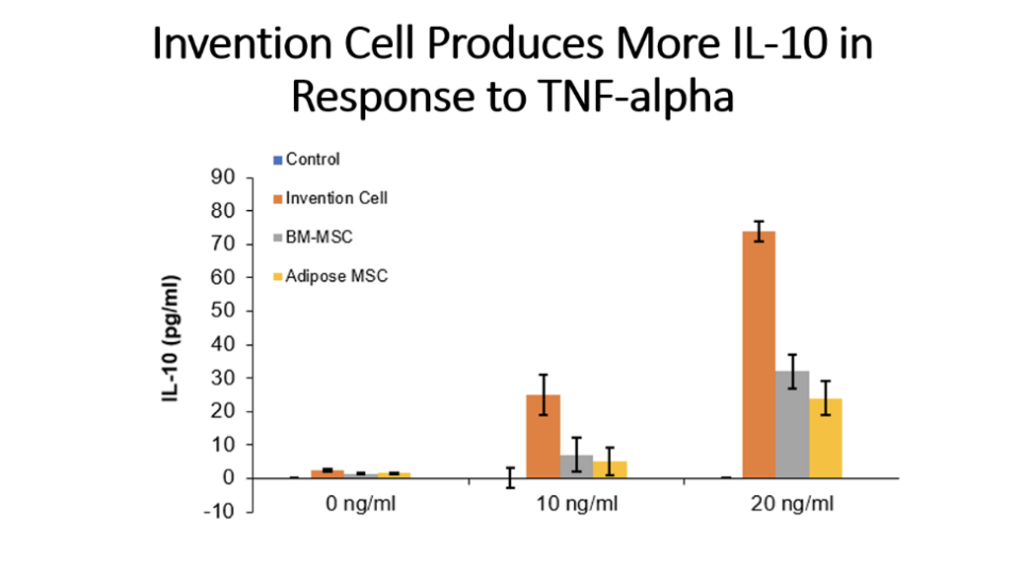
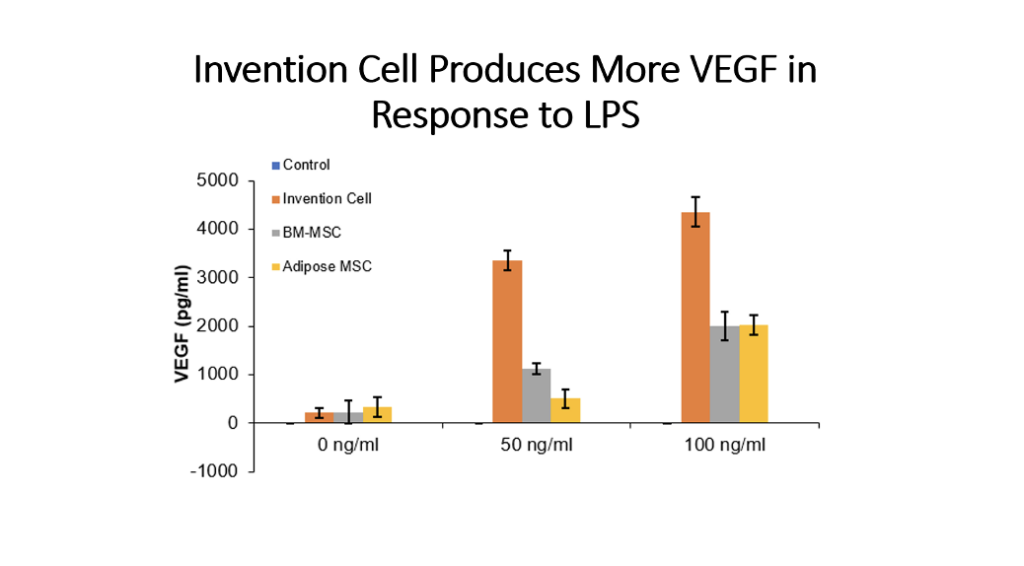
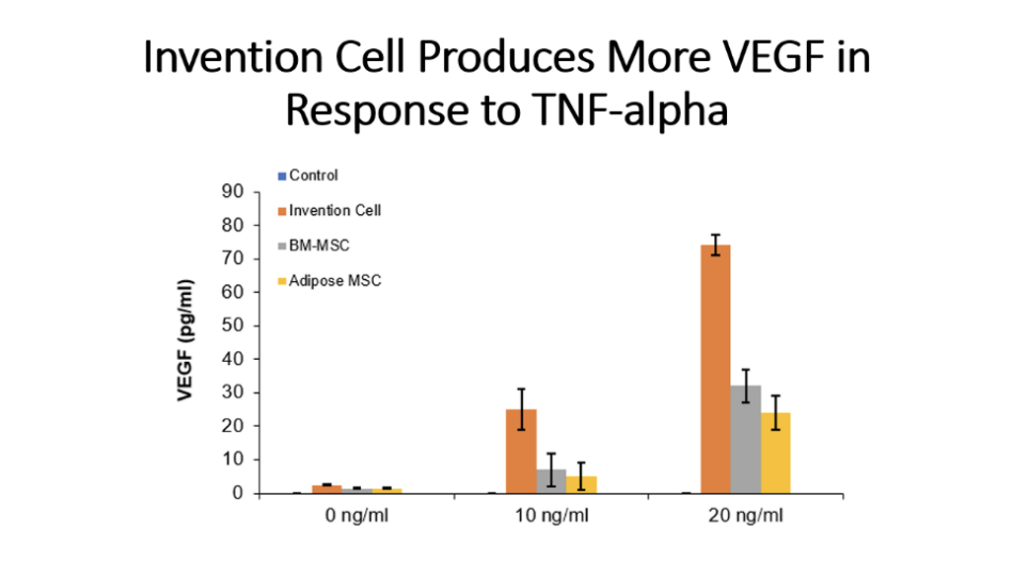
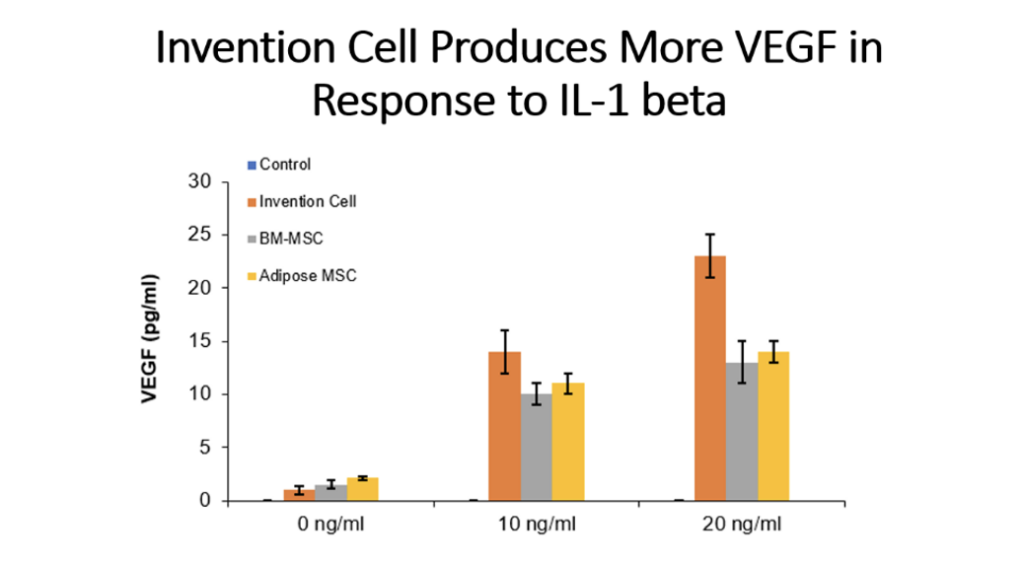
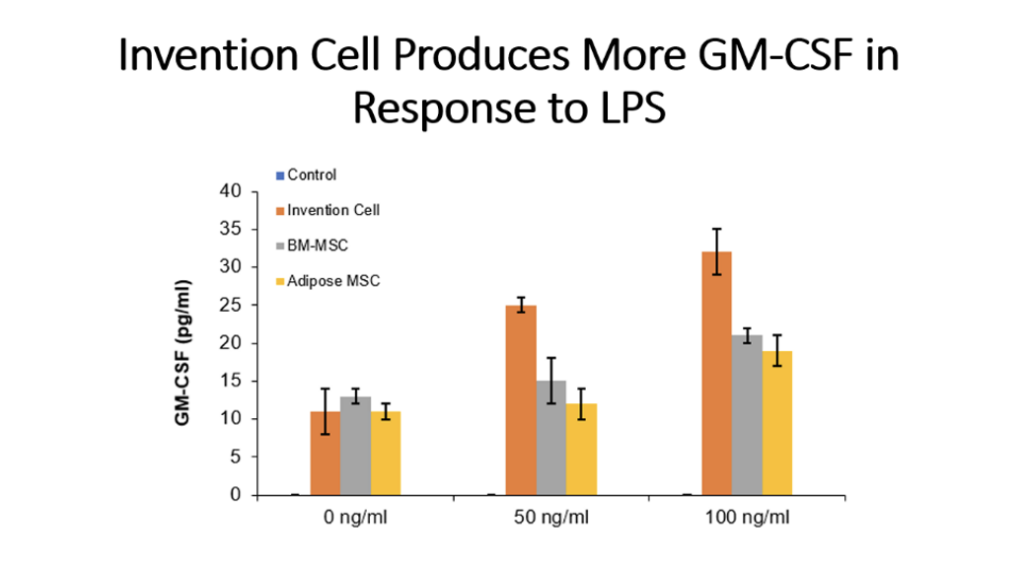
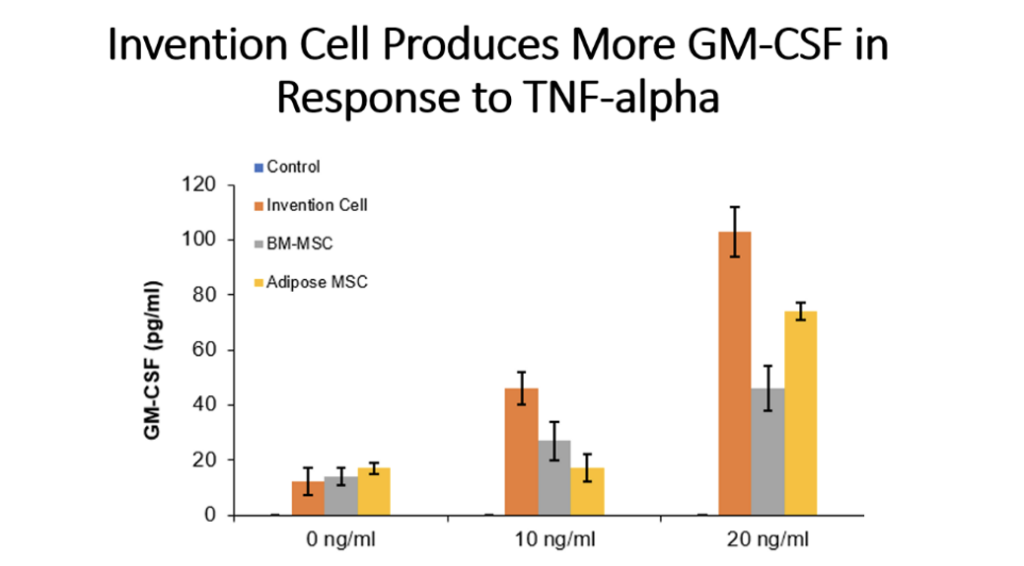
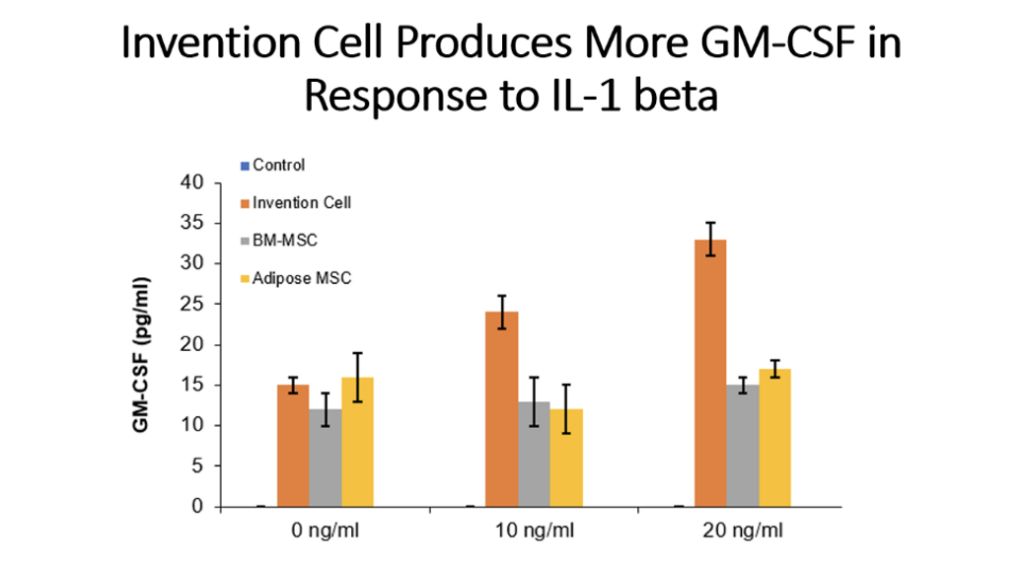
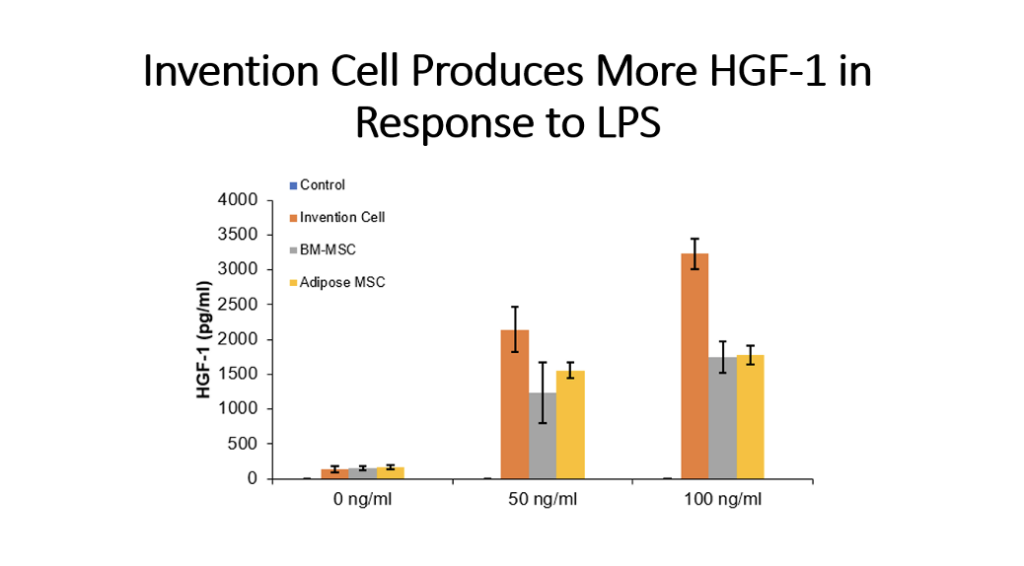
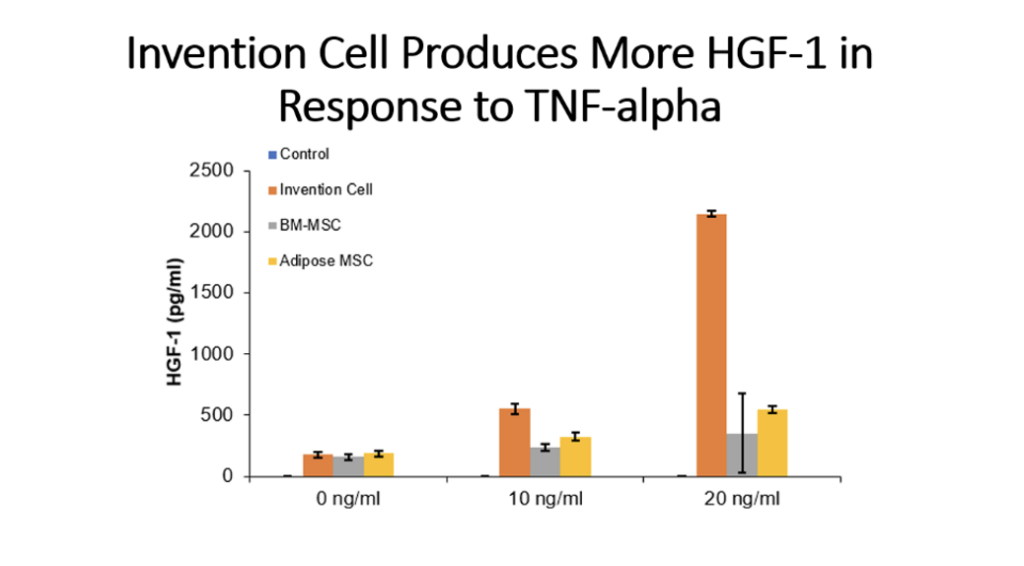
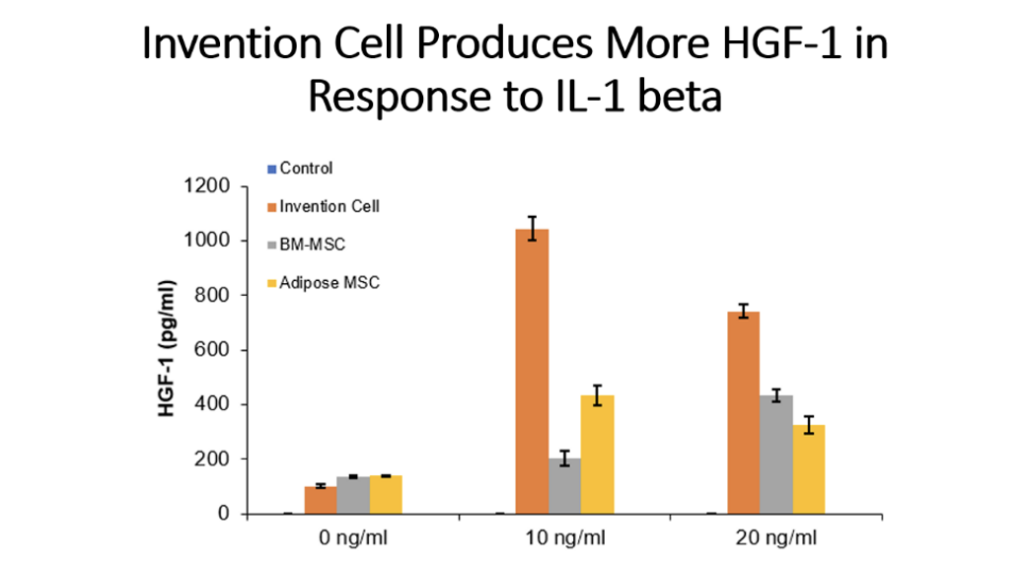
Example 5: Production of Cytokines by Cells of the Invention Compared to Bone Marrow MSC and Adipose Tissue MSC
Cells (Invention cells or BM-MSC or Adipose-MSC) were utilized at passage 5. Cells were cultured in standard in fully humidified environment with 5% carbon dioxide. Cultured in 96 well plates for 48 hours, 50,000 cells per well. Unstimulated or stimulated with 50 or 100 ng/ml LPS, 10 or 20 ng/ml TNF, 10 or 20 ng/ml IL-1 beta. Cytokines assessed by ELISA.